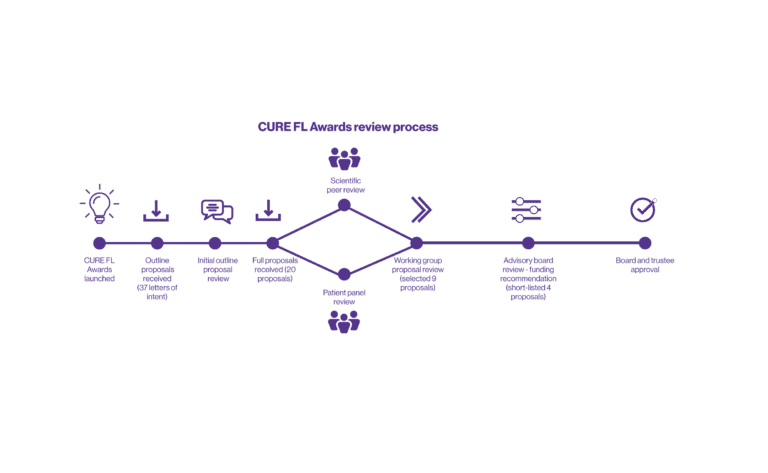
An Overview of the CURE FL Awards
What are the CURE FL Awards?
Our mission at the FLF is to lead new and determined efforts to find innovative treatments and cures for follicular lymphoma (FL). We have a suite of global initiatives aimed at making curative therapies available to FL patients as soon as possible, with our inaugural initiative being the CURE FL Awards –CUrative Research to Eliminate Follicular Lymphoma, launched in early 2022.
CURE FL Awards is a unique research grant program for the FLF, a catalyst for scientific research to lead to better treatments and cures. This program harnesses top clinical scientists and researchers from around the world, with the most innovative and exciting research projects. We hope our leading investigative teams will make strong progress and move quickly towards trials, and for successful outcomes to be available to patients as soon as possible.
The CURE FL Awards are a call for transformative projects in areas of treatment that we have identified as being the most likely to deliver rapid progress towards a cure. The FLF has worked closely with our partners, the Centre for Strategic Philanthropy (CSP) at the Milken Institute, to develop and launch the CURE FL Awards to assess and prioritise targets for philanthropic development for FL therapies. It aims to support innovative and impactful FL research with targeted funding.
The first cycle launched in early 2022 is run in collaboration with the Milken Institute, and the second cycle, launched in August 2023, is run in partnership with The Leukemia & Lymphoma Society and The Institute for Follicular Lymphoma Innovation.
Why are we doing this?
FL as a condition is often put in the “back seat” in research compared to other forms of lymphomas and blood cancers, because it is slower progressing, and clinical trials are more challenging. The CURE FL Awards are specifically designed to change this, to give an impactful voice to the FL community, to highlight that patients living with FL should not be overlooked and to drive the pace for new therapies, which are urgently needed.
Most FL patients experience several periods of remission and relapse over a number of years or decades. A small number of patients progress to a more aggressive form of FL earlier, with 5-year survival rates being as low as 50%. Even patients with more favorable survival rates endure both the psychological toll of an incurable, relapsing disease and the increasing toxicities of therapies designated for later relapses.
The CURE FL Awards are shining a light on FL and are catalyzing focused development of therapies for FL patients.al
“The CURE FL Awards mean real progress towards a cure. Speaking on behalf of our members, we feel supported – it helps us to worry less and look forward to a positive future. Wheels are in motion to find cures for us all, so thank you.”
Nicky Greenhalgh – Founder of the Living with Follicular Lymphoma community and patient representative.