
Technical abstract:
CAR-T cell therapy resistance may appear linked to antigen loss and/or an immunosuppressive tumor microenvironment (TME). We hypothesized that dual targeting of CD19 in combination with CD70 or Folate Receptor β (FRβ) may prevent tumor escape in follicular lymphoma (FL) patients. The novel targets proposed are addressed to target FL-TME crosstalk, based on knowledge and know-how of the teams included in this proposal.
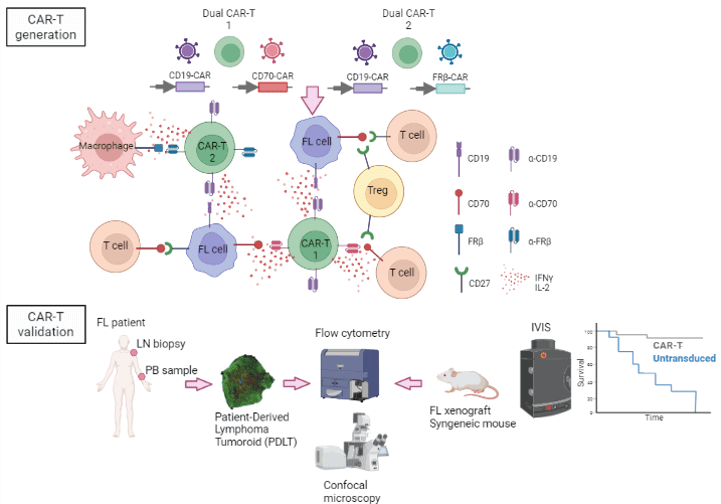
CD70 is highly expressed at diagnosis in FL tumor cells of patients that relapse or show a component of transformation to DLBCL. Moreover, CD70 tumor cells induce Treg generation, thus elimination of CD70+ cells may revert TME immunosuppression. FRβ is expressed in a subset of macrophages with an immunosuppressive M2-like profile (M2-Mφ). Repolarization or elimination of Mφ enhances immunotherapy efficacy in FL (Valero JG, Leukemia 2021). We have demonstrated the efficacy and safety of targeting M2- Mφ expressing FRβ with CAR-T cells in ovarian cancer (Rodriguez-Garcia A, Nat Comm 2021).
Our main goal is to develop novel tailored dual CD19-based CAR-T cells by exploring CD70 and FR-β as possible targets for the treatment of FL and compare their activity with CD19-CART (ARI-0001) developed in-house. The efficacy of dual CAR-T cells will be first tested in lymphoma cell lines expressing different antigen densities, and in Patient Derived Lymphoma Tumoroids (PDLT) generated from FL patient cells at relapse. These new in vitro systems include tumor B cell, autologous T cells and Mφ, and constitute a robust tool to examine ex vivo responses to immunotherapies. Finally, the efficacy and safety of the best dual CAR-T candidates will be characterized in animal models.
This project will use a comprehensive strategy combining our expertise in FL-TME and the generation of PDLT, and our extensive experience in the preclinical development of CAR-T cells. The bench-to-bedside translation will be secured as IDIBAPSHCB runs a unique clinical program with several academic CAR-T products for the treatment of hematological malignancies. Remarkably ARI-0001 is the first European-developed CAR-T therapy approved for its clinical use under hospital exemption. Upon the completion of this research project, we expect to have developed a new therapy that will be ready for testing in clinical trials in patients with follicular lymphoma. These pre-clinical experiments will be used to submit a formal application to the Spanish regulatory agency.
Name of applicants and institution
PI: Patricia Pérez-Galán, PhD. Group leader at the Institut d’Investigacions Biomèdiques August Pi I Sunyer (IDIBAPS), Barcelona, Spain
Co-PI 1: Sonia Guedan, PhD. Principal Investigator IDIBAPS.
Co-PI 2: Armando López-Guillermo, MD PhD. Group leader and Senior consultant, Fundació Clínic per a la Recerca Biomèdica (FCRB), Hospital Clínic de Barcelona (HCB), Barcelona, Spain
Co-PI 3: Alvaro Urbano-Ispizúa, MD PhD. Group leader and Director of Hemato-oncology Institute, FCRBHCB
IDIBAPS: Alba Rodriguez-García (post-doc) for CAR-T generation and Ferran Araujo (pre-doc) for ex vivo CAR-T testing in PDLT.
FCRB: Manel Juan MD, PhD and Julio Delgado MD, PhD for CAR-T manufacturing and application to clinical trials, respectively.